Forschung
Biothermofluidics for Cerebrospinal Fluid Diagnostics and Control
A variety of diseases, such as hydrocephalus and meningitis, are connected with pathological changes of the cerebrospinal fluid (CSF). Although a significant research effort has been undertaken by medically oriented research groups worldwide on the biochemistry of the cerebrospinal fluid, there is surprisingly little information available on its actual flow and related transport phenomena. This research project constituted the first synergistic effort to bring together the expertise of a number of very diverse disciplines. They assembled the power of computational simulation, magnetic resonance imaging (MRI), in vivo MRI-based flow and motion measurements and laser-based in vitro velocity measurements in order to piece together a complete picture of the flow of and mass transport within the CSF.
Introduction
The cerebrospinal fluid (CSF) is contained within and surrounds the brain and spinal cord. It suspends the brain through its buoyancy force and protects it from impact on the cranial vault walls in cases of sudden head motion. The CSF further serves as an intermediary between blood and nervous tissue, providing the latter with nutrients and removing waste products. Recent research shows that the cerebrospinal fluid flow is much more important than previously believed. For example, the pituitary gland and hypothalamus communicate through the CSF and new neurons follow the flow of cerebrospinal fluid in the adult brain. In order to better understand these phenomena, to improve existing procedures and devise new ones for the treatment of diseases of the cerebrospinal fluid space, a better understanding of CSF flow is invaluable. The only truly non-invasive way of measuring cerebrospinal fluid flow is magnetic resonance imaging (MRI). But despite the fact that MRI units are constantly being improved, neither time nor spatial resolution are high enough to capture all the necessary flow details. However, by using MRI to acquire the anatomy of the CSF space and brain tissue motion, and by performing MRI measurements of CSF flow at selected sites, enough information can be gathered to reconstruct the entire flow field using computational fluid dynamics (CFD) simulations. The validation of the reconstructed flow field can be performed using particle tracking velocimetry (PTV) on a transparent phantom.
In Vivo MRI Measurement
The classical approach to the modelling of the cerebrospinal fluid flow has been the extensive simplification of the anatomical structures of the CSF space. This approach was driven by the fact that neither the necessary computer power nor the MRI sequences needed for a subject-specific investigation were available until recently. Therefore, it was not possible to assess local flow features that are connected to variations of the local anatomy. The subject-specific approach taken in this project made it possible to consider those variations. Magnetic resonance imaging (Figure 1) constitutes the foundation for the subsequent modelling, providing anatomy as well as CSF flows and brain motion data.
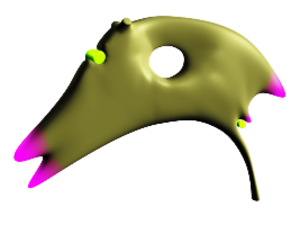
Image Processing
Data acquired with MRI has to undergo extensive processing before it can be used as base for the computational simulation of CSF flow. In a first step, the anatomy of interest has to be segmented from the MRI data (Figure 3). A surface reconstruction is then performed to obtain a parametric description of the domain (Figure 2). In order to reconstruct the velocity profile at prominent locations within the domain, the mass flow rate is calculated from the MRI velocity data and a high-resolution flow field that can be used for the subsequent simulations is obtained.
Computational Simulation
Computational fluid dynamic (CFD) simulations have become an indispensable tool for the design of airplanes, automobiles and other machinery where fluid flow is of importance. The equations that govern the air flow around the wing of an airplane can also be used to calculate the cerebrospinal fluid flow in the brain of a human (Figure 4 and Figure 5). While turbulence is the main challenge in the former application, the complexity of the system geometry and its intricate deformation are the main challenges in the latter.
In Vitro PTV Measurement
While computational fluid dynamics simulations are a reliable tool to calculate flow and pressure fields, their use in the novel application area of cerebrospinal fluid flow still calls for an initial validation. Because of the limited resolution of MRI and the lack of other tools that can be used to reliably measure CSF flow in vivo, an in vitro approach has to be followed. The parametric description of the anatomy obtained by segmentation and surface reconstruction of the MRI data can be used to build a transparent phantom that has the same geometry as the model used for the CFD simulations (Figure 6). Using 3D particle tracking velocimetry (3D), the flow field in the phantom can be measured and compared to the CFD calculations (Figure 7). The MRI-compatible construction of the phantom and of the apparatus driving the flow within the phantom also allows for a cross-validation with MRI velocimetry.
Videos
The following video shows the tracks of virtual massless particles following the 3D flow of CSF in a human subarachnoid space over several cardiac cycles [1].
The next video shows a 3D visualization of CSF flow velocity vectors in a human subarachnoid space during one cardiac cycle [1].
Acknowledgements
– Financial support for the former project was provided by the ETH Zurich Research Commission.
– The anatomy acquisition was carried out at the Institute of Neuroradiology, University Hospital Zurich. The flow velocity and brain motion measurements were carried out at the Institute for Biomedical Engineering, UZH and ETH Zurich.
– Image processing was performed at the Computer Vision Laboratory, ETH Zurich and the Laboratory of Thermodynamics in Emerging Technologies, ETH Zurich.
– The computational fluid dynamics simulations were carried out at the Laboratory of Thermodynamics in Emerging Technologies, ETH Zurich.
– The phantom and the driving apparatus were developed by the Measurement and Control Laboratory, ETH Zurich. The 3D PTV measurements were carried out at the Institute of Environmental Engineering, Chair of Groundwater and Hydrodynamics, ETH Zurich. The MRI measurements were performed at the Biomedical Engineering, UZH and ETH Zurich.
Publications
- S. Gupta, M. Soellinger, D. Grzybowski, P. Boesiger, J. Biddiscombe, D. Poulikakos & V. Kurtcuoglu. Cerebrospinal fluid dynamics in the human cranial subarachnoid space: an overlooked mediator of cerebral disease. I. Computational model. Journal of the Royal Society Interface 7, 1195-1204, 2010.
- D.W. Holman, V. Kurtcuoglu & D.M. Grzybowski. Cerebrospinal fluid dynamics in the human cranial subarachnoid space: an overlooked mediator of cerebral disease. II. In vitro arachnoid outflow model. Journal of the Royal Society Interface, 2010. doi: 10.1098/rsif.2010.0032.
- Kurtcuoglu V, Gupta S, Siyahhan B, Poulikakos D. Modeling of flow in the cranial subarachnoidal cerebrospinal fluid space. 6th World Congress of Biomechanics, Symposium on Cranial CSF Mechanics 2010, Singapore, August 1-6
- Kurtcuoglu V. Modeling of flow in the cerebrospinal fluid space – challenges and opportunities. The University of Western Australia, School of Mechanical and Chemical Engineering Seminar 2010, Perth, Australia, July 27
- Gupta S, Soellinger M, Boesiger P, Poulikakos D & Kurtcuoglu V. Three-dimensional computational modeling of subject-specific cerebrospinal fluid flow in the subarachnoid space. J Biomech Eng, 131(2):021010, Feb 2009. doi:10.1115/1.3005171.
- Schibli M, Wyss M, Boesiger P & Guzzella L. Investigation of ventricular cerebrospinal fluid flow phase differences between the foramina of monro and the aqueduct of sylvius. Biomed Tech (Berl), 54(4):161–169, Jul 2009. doi: 10.1515/BMT.2009.021.
- Soellinger M, Rutz AK, Kozerke S & Boesiger P. 3D cine displacement-encoded MRI of pulsatile brain motion. Magn Reson Med, 61(1):153–162, Jan 2009. doi:10.1002/mrm.21802.
- Schibli M, Wiesendanger M, Guzzella L, Hoyer K, Soellinger M, Kurtcuoglu V & Boesiger P. In-vitro measurement of ventricular cerebrospinal fluid flow using particle tracking velocimetry and magnetic resonance imaging. In Proc. First International Symposium on Applied Sciences on Biomedical and Communication Technologies ISABEL ’08, 1–5. 25–28 Oct. 2008. doi:10.1109/ISABEL.2008.4712622.
- Kurtcuoglu V, Soellinger M, Summers P, Boomsma K, Poulikakos D, Boesiger P & Ventikos Y. Computational investigation of subject-specific cerebrospinal fluid flow in the third ventricle and aqueduct of Sylvius. J Biomech, 40(6):1235–1245, 2007. doi:10.1016/j.jbiomech.2006.05.031.
- Kurtcuoglu V, Soellinger M, Summers P, Poulikakos D & Boesiger P. Mixing and modes of mass transfer in the third cerebral ventricle: a computational analysis. J Biomech Eng, 129(5):695–702, Oct 2007. doi:10.1115/1.2768376.
- Mandelkow H, Halder P, Brandeis D, Soellinger M, de Zanche N, Luechinger R & Boesiger P. Heart beats brain: The problem of detecting alpha waves by neuronal current imaging in joint EEG-MRI experiments. Neuroimage, 37(1):149–163, Aug 2007. doi:10.1016/j.neuroimage.2007.04.034.
- Miyati T, Mase M, Kasai H, Hara M, Yamada K, Shibamoto Y, Soellinger M, Baltes C & Luechinger R. Noninvasive MRI assessment of intracranial compliance in idiopathic normal pressure hydrocephalus. J Magn Reson Imaging, 26(2):274–278, Aug 2007. doi:10.1002/jmri.20999.
- Soellinger M, Ryf S, Boesiger P & Kozerke S. Assessment of human brain motion using CSPAMM. J Magn Reson Imaging, 25(4):709–714, Apr 2007. doi:10.1002/jmri.20882.
- Kurtcuoglu, V., Poulikakos, D., Ventikos, Y. Computational Modeling of the Mechanical Behavior of the Cerebrospinal Fluid System. ASME Journal of Biomechanical Engineering 2005; 127(2): 264-9.
- Kurtcuoglu, V., Soellinger, M., Summers, P., Boomsma, K., Poulikakos, D., Boesiger, P., Ventikos, Y. Reconstruction of cerebrospinal fluid flow in the third ventricle based on MRI data. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv 2005; 8(Pt1): 786-93.